How to Use SECO DNEL Tool to Calculate REACH DNELS
Little Pro on 2018-11-15
Derived No-Effect Levels (DNELs) are levels of exposure above which humans should not be exposed. They are used in quantitative chemical risk assessment. Exposure/DNEL<1 means that the risk of a chemical substance to humans can be considered to be controlled/acceptable. In this article, we will show you how to calculate DNELs using Simple European Calculator of DNEL (SECO-DNEL Tool).
Challenges with DNEL Calculation
If you have some knowledge of chemical risk assesment already, you should know that DNEL = NOAEL/AF. NOAEL is the No Observed Adverse Effect Level obtained from toxicology studies. AF refers to assessment factor with a range of 10 to 10,000.
The real challenge with DNEL calculation is that it is not a single value. You need to derive a DNEL for each relevant exposure pattern (population, route, and duration of exposure) and each relevant health effect (local and systemic effects).Together, there are more than a dozen of possible DNEL values for just 1 substance (see table below). The other change is that you need to modify dose descriptors if you do not have NOAEL for a specific route. For example, you need to convert an oral NOAEL rat (in mg/kg bw/day) to a corrected inhalatory NOAEC (in mg/m3) first in order to derive DNEL values for inhalation routes.
| Exposure Pattern | Workers | General Population |
|---|---|---|
| Acute – inhalation, systemic effects | ||
| Acute – dermal, local effects | ||
| Acute – inhalation, local effects | ||
| Long-term – dermal, systemic effects | ||
| Long-term – inhalation, systemic effects | ||
| Long-term – oral, systemic effects | X(not relevant) | |
| Long-term – dermal, local effects | ||
| Long-term – inhalation, local effects |
What is SECO DNEL Tool and How to Download
The Simple European Calculator of DNEL (SECO-DNEL Tool) is free excel-based tool developed by the Section of Chemicals and Occupational Health (SECO) of Swiss authority. It can be used to calculate DNELs for all possible scenarios. Modifying dose descriptor is also handled in the background.
To download the tool, please click the link below.
How to Use SECO DNEL Tool
SECO DNEL Tool is relatively easy to use. All you need to do is to follow the 3 steps listed below.
- Step 0: Enter substance details
- Step 1: Select the relevant dose descriptor for the endpoint concerned (e.g. NOAEL, LOAEL)
- Step 2: Modify, when necessary, the relevant dose descriptor per endpoint to the correct starting point
- Step 3: Apply, when necessary, assessment factors to the correct starting point
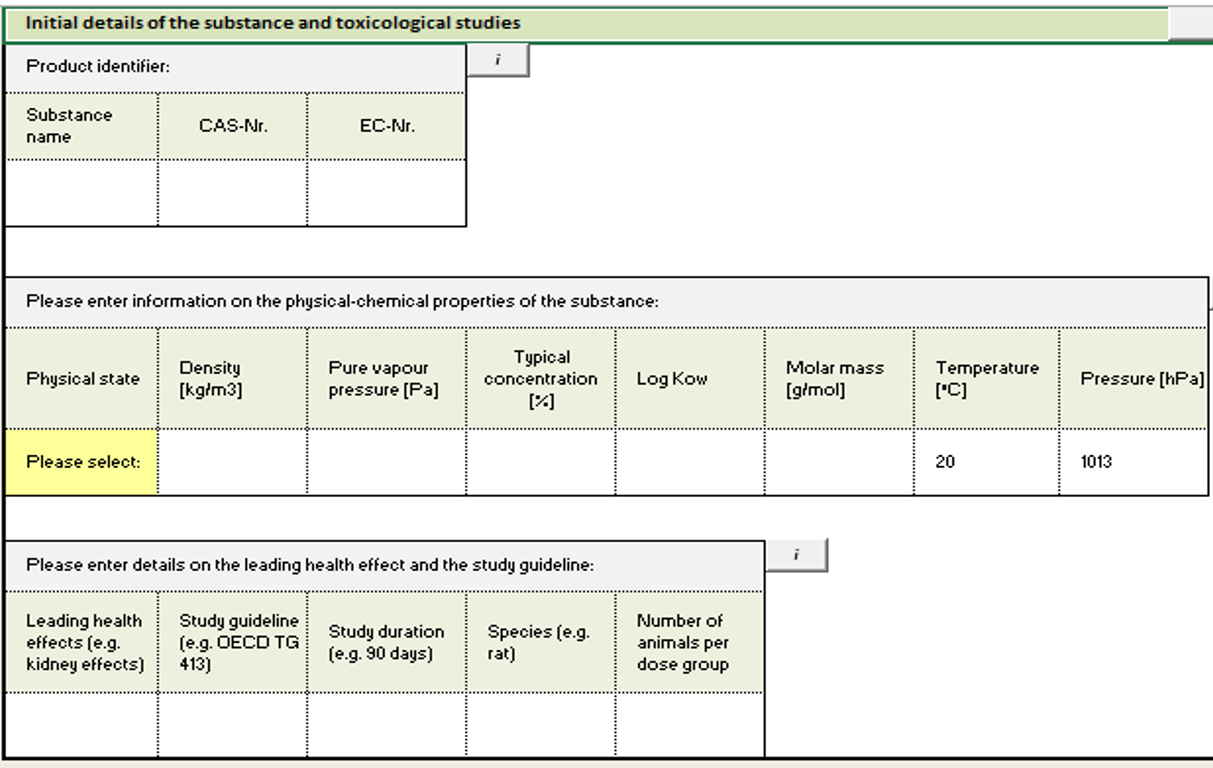
Step 0: Enter substance details
In this step, you enter substance identification info, basic physico-chemical properties(see picture below) and provide leading health effects.
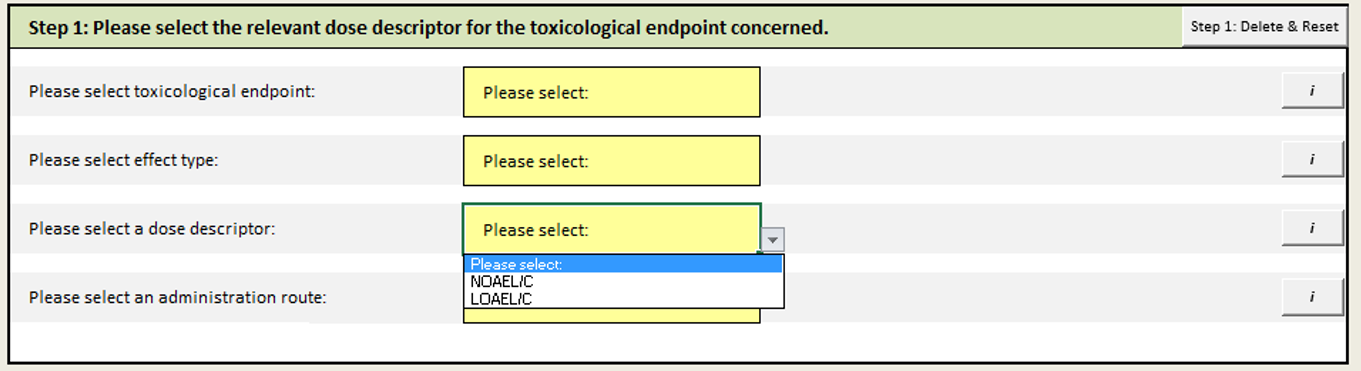
Step 1: Select relevant dose descriptors (NOAEL, LOAEL)
In this step, you need to specify the type of health effects( reprotox effects vs other effects, local effects vs systemic effects) and provide available dose descriptors (NOAEL, LOAEL) and administration route.
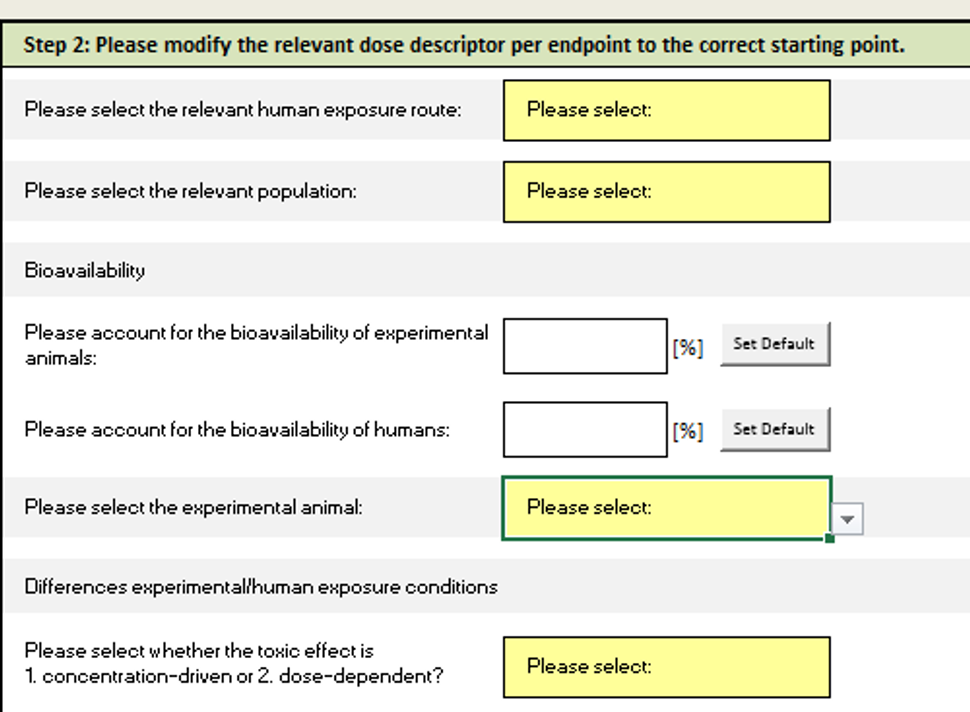
Step 2: Modify dose descriptors
In this step, you need to provide human exposure routes, tested animals and the bioavailability difference between humans and animals.
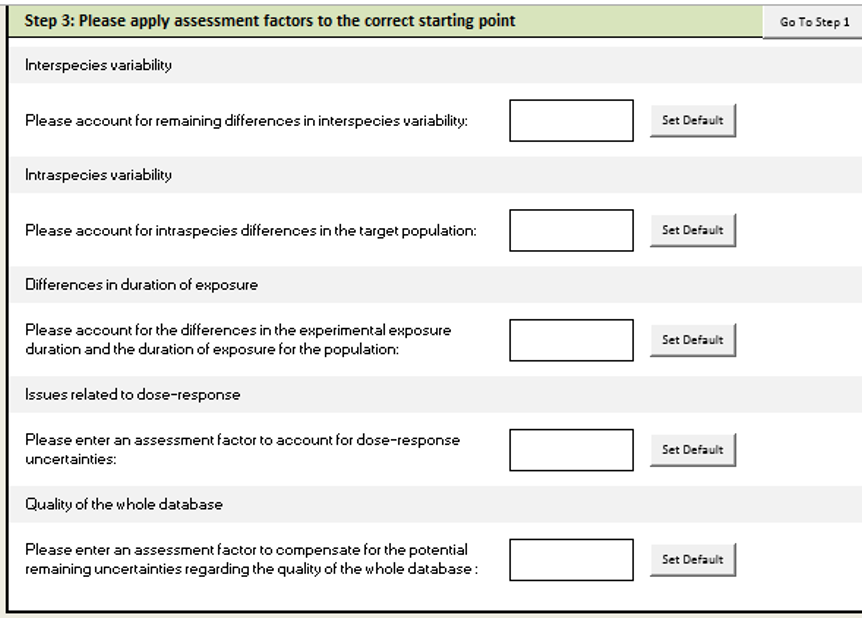
Step 3: Enter assessment factors
You can click "set default" to use default assessment factors. You can also override the default values by entering assessment factors manually. Using default value is recommended.
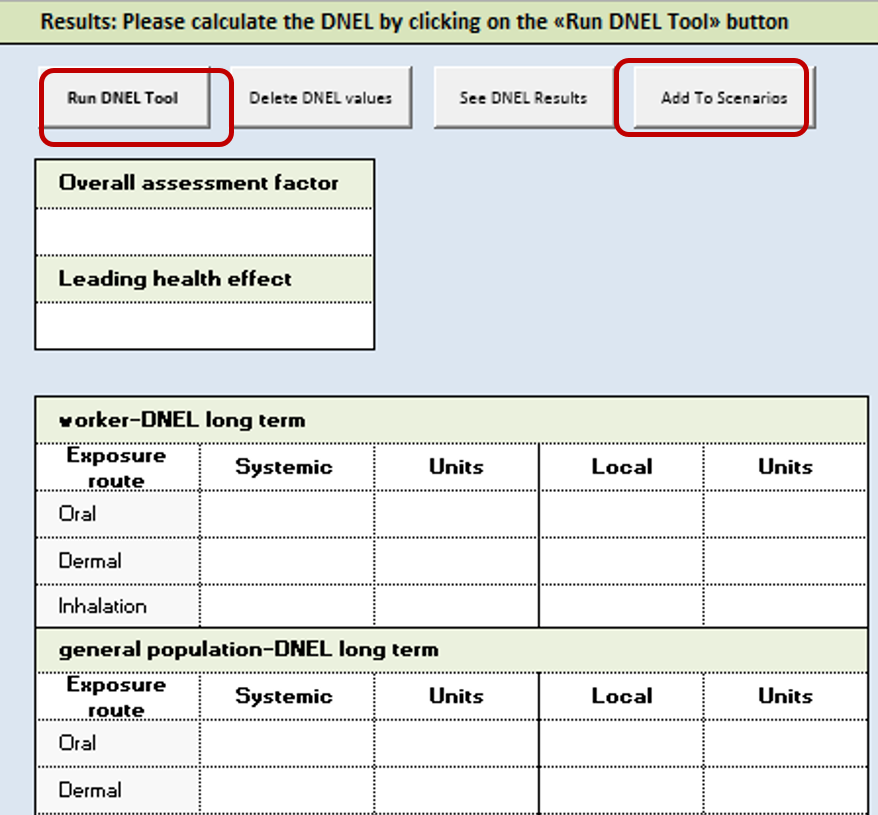
Presentation of Calculation Results
Once you have entered all required information, you can click "Run DNEL tool". The calculated DNEL values will be given in cell marked in yellow (see picture below). Congratulations that you have got the first DNEL value for 1 scenario. If you are happy with the result, you can click "Add to scenario" and repeat step 1, 2 and 3 for other possible scenarios.
Reference
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..
