How to Use ECETOC TRA Tool Part II: Consumer Exposure Estimation and Health Risk Assessment
Little Pro on 2017-02-09
Consumers may be directly or indirectly exposed to certain chemical substances from the products they use, for example, ingredients in cleaning products, solvents in glues or dyes in textiles. We would like to know whether such chemical exposure poses unacceptable risks to consumers or not. In this article, we will show you how to use ECETOC's Targeted Risk Assessment tool (ECETOC TRA) to conduct consumer exposure estimation and consumer health risk assessment.
If you do not know what ECETOC TRA tool is, how to download it and its general workflow, please read the following tutorial first.
Procedure of Consumer Health Risk Assessment
Before we start using the ECETOC TRA tool, it is very important to know the procedure of consumer health risk assessment and when the tool is needed.
| Step | Tasks |
|---|---|
| Hazard Characterization |
Determine GHS hazard classification and the type of adverse health effects (acute or chronic effects, or local/systemic effects.) Derive no-effect levels for human health (DNEL) or DMEL by taking into account the foreseeable routes of exposure and duration of exposure (chronic, chronic adjusted for shorter daily duration, or infrequent use). Note: The ECETOC TRA tool cannot be used to derive GHS classification or calculate DNELs/DMELs. You have to do this manually. DNEL values will be used as input parameters later. |
| Exposure Assessment |
Describe product category or article category using standard descriptors and collect info on use conditions based on consumer habits and practice. Use ECETOC TRA tool to calculate exposure estimates for potential routes of exposure (inhalation, dermal or oral) and each applicable phase of activity (preparation, application, post-use exposure and cleaning/removal). |
| Risk Characterization |
Risk characterization ratio (RCR) = Exposure Estimate/DNEL (per use and route). This can be done in ECETOC TRA if DNEL values are provided. • RCR<1, acceptable risk; • RCR>1, unacceptable risk. |
Principles of ECETOC TRA's Consumer Exposure Estimation
The tool has built in common exposure models and very conservative assumptions (see table below). We have also listed key factors influencing consumer exposure levels.
| Scenario | Assumption | Key factors |
|---|---|---|
|
Inhalation: A substance is released into a standard room as a gas, vapour or airborne particulate, or by evaporation from liquid product or solid matrices like articles (e.g. solvent from painted walls). |
The substance is released instantaneously into a standard room with immediate mixing with air, and no removal takes place due to ventilation. 100% is released for absorption. Default room size is 20m3. Default body weight is 60kg. |
|
|
Dermal case 1: Exposure to a substance contained in a mixture (non-continuous immersion such as direct application, dipping, splashing.) |
All the substance contained in a skin contact layer of 0.01cm thickness will be available for absorption. Default dermal absorption rate is 100%. Default body weight is 60kg. Default density of directly applied product: 1,000g/L. Dilution factor=1 (if not diluted when use). |
|
|
Dermal case 2: Exposure to a substance migrated from article matrixes (i.e, toys, clothes) |
All the substance contained in a skin contact layer of 0.001cm~0.01cm thickness (depending on article) will be available for absorption. Default layer thickness: 0.001cm Default dermal absorption rate is 100%. Default body weight is 60kg. |
|
|
Oral case 1: Exposure of a substance during nomal use (including hand to mouth transfer) |
The dose of ingested substance depends on the total amount of product swallowed and substance weight fraction in product. Default absorption rate is 100% Default hand to mouth transfer rate is 100%. Default body weight is 60kg. |
|
|
Oral case 2: Ingestion of a substance migrated from article matrix (i.e, licking) |
The dose of ingested substance depends on the volume of product swallowed and substance weight fraction in product. The volume of product swallowed is calculated based on the article area in contact with the mouth(default 10 cm2) and the thickness of article layer thickness assumed to be in contact during mouthing (default value 0.001 cm). Default transfer and ingestion rate is 100%. Default body weight is 60kg. |
|
Workflow of ECETOC TRA Tool and Exercise
The picture below shows you the workflow of ECETOC TRA tool. To start with, you need to input substance identification info (step 1) and basic physio-chemical properties (step 2). In step 3b, you will need to input consumer exposure info and no-effect levels or reference values. In step 4, you can run the tool and view results. Since we focus on consumer risk assessment, we will skip 3a and 3c in this tutorial.

Let's assume that we need to assess ethanol's risk to consumers for the use of a cleaning product containing 20% ethanol by weight. The table below summarizes the input parameters for ECETOC TRA tool in this exercise. The minimum required input parameters are marked in bold.
|
Step 1 Enter substance identification |
|
|
Step 2 Enter phsyio-chemical properties |
|
| Step 3b Enter use scenario info |
Once a product category has been assigned, ECETOC TRA automatically sets default values for the following parameters based on conservative use habits. However, these values can be overwritten for refined assessments. Justifications need to be provided.
Transfer factor is the actual percentage of a substance available for release. For example, during tank filling with 20L of gasoline, not all these 20L are available to be released into air. Default values associated with product categories, such as amount of product used per application and exposure time (for the inhalation route), were obtained from the RIVM fact sheets for specific products. |
| Step 3b Enter reference values for consumers (DNELs) |
|
In the following part of this article, we will show you how to input above info into the tool and get estimated consumer exposure levels and risk characterization result.
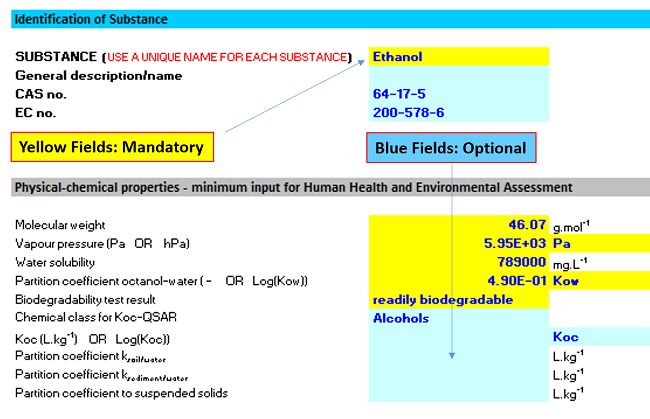
ECETOC TRA Tool Step 1 and Step 2
The picture below shows you the step 1 and step 2 input in this practice.
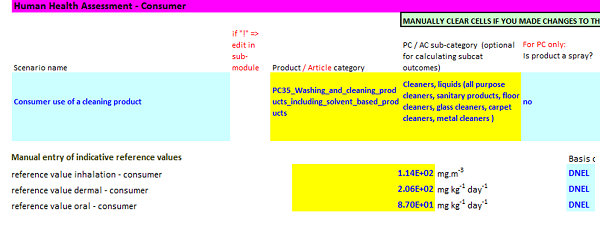
ECETOC TRA Tool Step 3b
The picture below shows you how to input consumer exposure scenario info and reference values (DNELs) to the ECETOC TRA tool.
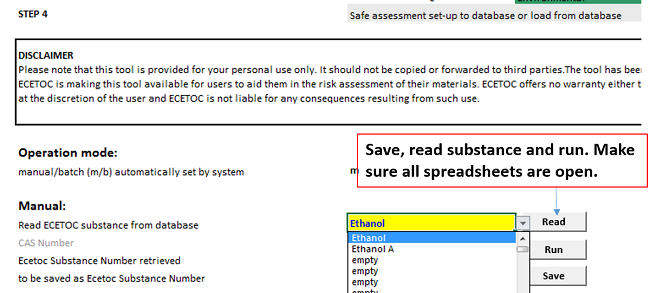
Step 4 It's Time to Run the Tool and See the Result!
After you have input all required information in yellow fields, you can follow the instructions below and run the tool. All other spreadsheets are also important for background calculations. Please make sure all spreadsheets are open when you save a substance, read substance and run the ECETOC TRA tool. Otherwise, you will get this error: Run-time error "9": Subscript out of range.
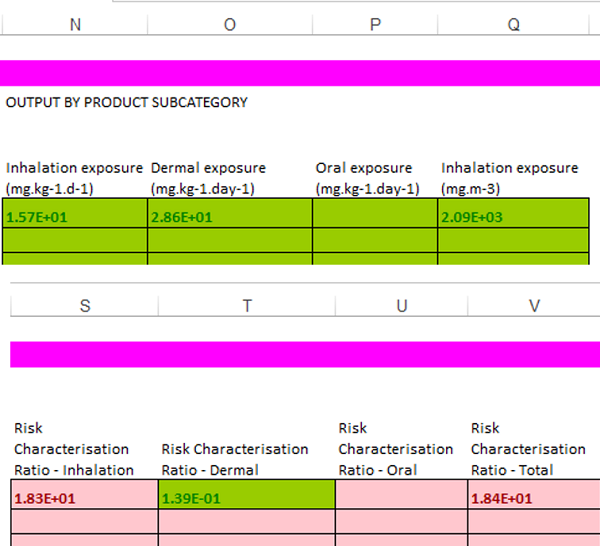
After you have clicked "run", you wait for about 30 seconds. Then the estimated consumer exposure levels and risk characterization results will be shown in column N-V (see example below). Green color means that the risk is acceptable while purple color means that the risk is not controlled.
What If Risk Is Not Controlled?
If the risk is not controlled, you can only do a limited number of things to control consumer exposure levels(i.e, lowering substance content in product, offering product in small packages) since consumers are usually not good at following use constructions or wearing personal protective equipment.
Please note that the ECETOC TRA tool only provides tier 1 screening exposure estimates (rather than realistic exposure estimations). Higher models such as ConsExpo are recommended for refined assessment if tier 1 risk assessment fails.
Reference

Good job. You have learned how to use ECETOC TRA tool to do consumer health risk assessment. Now go and pratice it. Please subscribe our newsletter to keep updated of our new articles.
"It does not matter how slowly you go as long as you do not stop. "
– Confucius
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..
