Turkish CLP Regulation: Regulation on Classification, labelling and Packaging (CLP) of Dangerous Substances and Preparations
Little Pro on 2016-01-05
The Turkish Ministry of Environment and Urbanization has issued revised Regulation on Classification, labelling and Packaging (CLP) of Dangerous Substances and Preparations in their Official Gazette with number 28848 on 11 December 2013. This regulation, also known as SEA regulation in Turkish, is aligned with EU CLP Regulation (1272/2008 EC) and fully enters into force on 1 June 2016.
The regulation sets detailed rules and principles for chemical classification, labelling and packaging in Turkey and brings GHS concepts to Turkey. Similar EU CLP regulation, it also requires manufacturers and importers to notify the classification and labeling of hazardous substances and mixtures to Turkish C&L Inventory ("C&L Notification").
Obligations of Different Roles under Turkish CLP Regulation
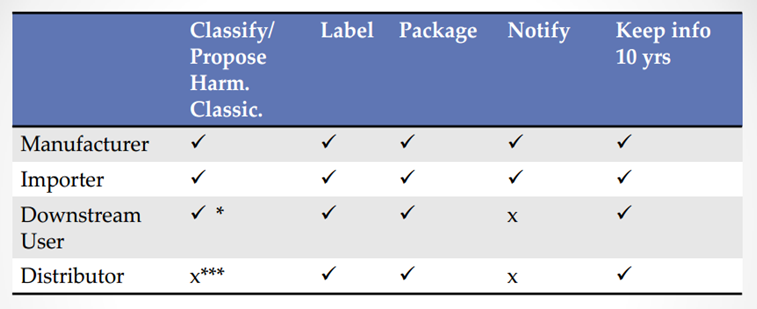
The picture below shows obligations of different roles under Turkish CLP regulation:
Critical Dates of Turkish CLP Regulation
| 1 June 2014 - 1 June 2015 |
|
| Since 1 June 2015 |
|
| Since 1 June 2016 |
|
For products already on the market prior to above date, an additional two-year grace period is given to avoid re-labelling.
Only Representative
Exporters can submit notifications through a Turkish Legal Representative to keep their business confidential.
Exemptions of Turkish CLP Regulation
The Turkish CLP regulation does not apply to:
- Medical products, veterinary medicinal products, cosmetic products, medical devices, and food or feeding stuff in finished states and intended for final user;
- Radioactive substances and mixtures;
- Non-isolated intermediates;
- Substances for research & development;
- Wastes;
Classification and Labelling Requirements in Turkey
Reference & Resources
Click here to access all references and resources for Turkey including the English translation of regulations, regulatory lists and useful links to the websites of competent authorities.
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Turkey, Resources and References