Taiwan TCSCA Registration
Little Pro on 2015-12-31
Under TCSCA, enterprises manufacturing or importing new substances or a given quantity of existing chemical substances shall register those substances with the Environmental Protection Administration (EPA). Enterprises that manufacture or import new substances or existing toxic chemical substances without the approval of the EPA will be liable to fines in the range of NT$200,000-2 million for manufacturers or NT$30,000-300,000 for importers.
On 5 Dec 2014, EPA issues Regulations on New and Existing Substance Registrations under TCSCA. This regulation has set out detailed information requirements for chemical registrations under TCSCA. In this post, we will take a look at the new regulation and help you understand the types of registration and data required for different types of registrations. Since a new substance also requires notification under the Occupational Safety and Health Act(OSHA), we have compared new substance notification under TCSCA with new substance notification under OSHA.
Overview of Substance Registrations under TCSCA
Under TCSCA, registrants are domestic manufacturers(M) and importers(I) of chemical substances. Domestic manufacturers and importers can appoint third-party representatives to handle substance registrations via notarized appointment letters. Foreign companies cannot submit substance registrations by appointing a local only representative.
| Category | Registration Type | Who/When |
|---|---|---|
| Registration of New Substance |
|
|
| Registration of Existing Substances |
|
|
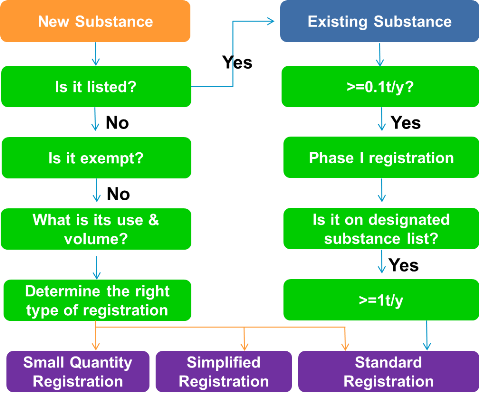
The picture below shows how to determine if a new substance or existing substance requires registration under TCSCA.
Registration of New Substances - Exemptions
The following new substances are exempt from registrations under TCSCA.
- Naturally occurring substances;
- Chemical substances accompanied in machines and equipment for test-run purposes;
- Non-isolated intermediates;
- Chemical substances for national defense purposes or under customs supervision;
- Waste;
- Mixture(only applicable to a substance in a mixture);
- Article(only applicable to a substance intended to be released from an article);
- Polymer meeting 2% rule with all monomers listed;
- Chemicals subject to other laws such as pesticides, environmental agents, pharmaceuticals, cosmetics, food additives, etc.
Registration of New Substances - Types of Registration and Requirements
There are three types of registrations for new substances under TCSCA depending on the uses and volume of the new substances: small quantity registration, simplified registration and standard registration.
The picture below shows how to determine which type of registration is required for a new substance.
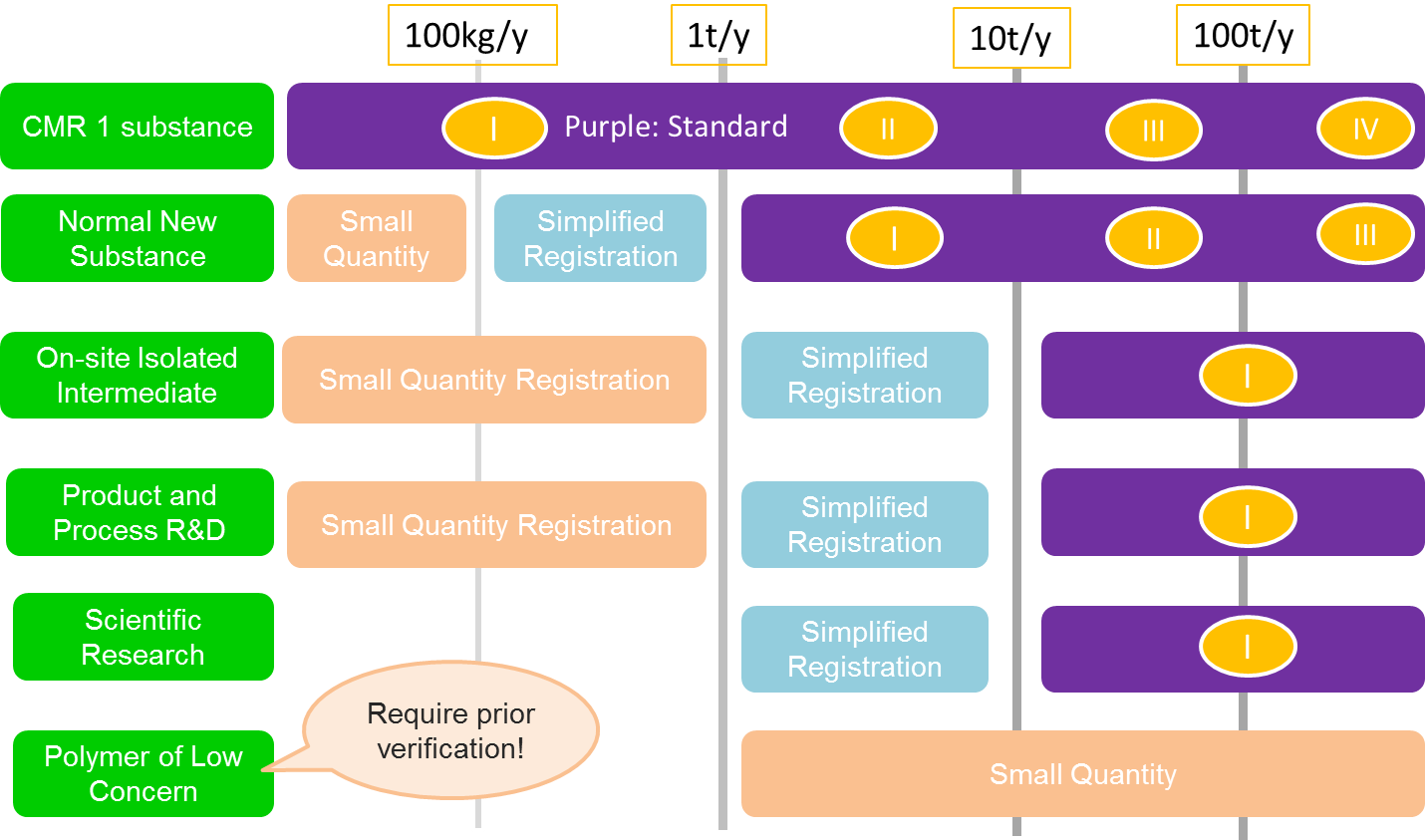
The table summarizes information requirements for above 3 types of registration.
| Small Quantity Registration |
|
| Simplified Registration |
|
| Standard Registration |
|
Detailed data requirements are set out in Regulations of New and Existing Substance Registrations.
Registration of New Substance - Review Period and Validity of Registration Document
| Type | Review Period and Validity |
|---|---|
| Small Quantity Registration |
|
| Simplified Registration |
|
| Standard Registration |
|
New substances that are registered under standard registration or polymer of low concern registered under small quantity registration will be added to the inventory of existing substances 5 years after registration has been completed.
Registration of New Substances - Transitional Measures
The guidance has also set transitional measures for new substances manufactured in or imported into Taiwan before 11 Dec 2014 and between the period of 11 Dec 2014 to 11 Dec 2015.
| Before 11 Dec 2014 |
|
| Between 11 Dec 2014 & 31 Dec 2015 |
|
| Standard Registration |
|
Registration of Existing Substances - Phase I Registration
Phase I registration is equivalent to pre-registration under EU REACH regulation and it needs to be submitted individually. The information required is very simple and includes registrant's contact info, substance identification, quantity, and info on manufacture and uses. The purpose of Phase I registration is to gather information on substances circulated in Taiwan, help EPA designate existing substances subject to registration and assist joint submission of existing substance registrations.
Registration of Existing Substances - Standard Registration
EPA will announce the list of designated existing substances subject to standard registration in several batches. Each batch will be given a grace period of 2-3 years. The first batch of 106 existing substances has been published in March 2019. Data requirement is the same as standard registration of new substances.
Joint Submission
For both new substances and existing substances, co-registrants may apply for joint registration under agreement.
For joint registration that is agreed by co-registrants, but no agreement is reached upon the cost sharing of registration information, the competent authority may determine the cost to be equally shared at the request of the later co-registrants. Then use of registration information is approved after the shared cost has been paid.
New Substance Notification: OSHA vs TCSCA.
Both OSHA and TCSCA require a new substance to be notified prior to its production and importation. While the type of new substance notification is the same and required data is similar, there are some differences between notifying a new substance under OSHA and notifying a new substance under TCSCA. The table below summarizes the main differences between OSHA and TCSCA when it comes to new substance notification.
| Items | OSHA | TCSCA |
|---|---|---|
| Registration Target |
|
|
| Type of Notification |
|
|
| Standard Registration - Hazard Data |
|
|
| Chemical Risk Assessment |
|
|
| Transitional Measures |
|
|
| Registration Platform |
|
|
Currently Taiwan EPA acts as the single registration window to avoid the duplication of registration requirements based on TCSCA and OSHA laws.
Reference & Resources
Click here to access all references and resources for Taiwan including the English translation of regulations, regulatory lists and useful links to the websites of competent authorities.
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Taiwan, REACH-like Regulation and Registration