How to Comply with Amended K-REACH Regulation 2019
Little Pro on 2015-12-31
The amended Act on Registration and Evaluation, etc of Chemical Substances in South Korea (also known as K-REACH) was promulgated in March 2018 and will come into force on 1 Jan 2019. In this article we will give you an overview of the amended K-REACH regulation and summarize what the main changes are. We will also compare it with EU REACH regulation. [Last update: 17 Sept 2018].
Main Requirements of Amended K-REACH
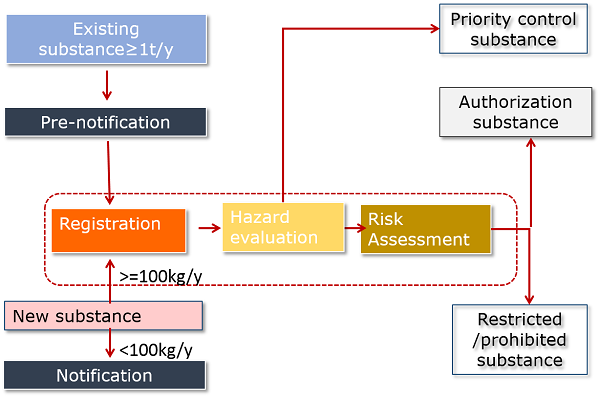
Under amended K-REACH, any person who intends to manufacture or import a new chemical substance or at least one ton per year of an existing chemical substance shall register the chemical substance ("registration") according to the following requirement:
- New substances must be registered prior to manufacture or import. <100kg/y new substances only require notification and do not need to go through hazard evaluation.
- All >=1t/y existing chemical substances (excluding exempt substances) must be registered within given grace periods.
- To benefit from the grace periods for existing substances, manufacturers and importers of >=1t/y existing chemical substances must notify their company info, substance name, volume, classification and use info to the MoE in advance ("pre-notification").
Foreign manufacturers who export chemical substances to Korea may appoint a Korea-based only representative to submit pre-notification or registrations.
In addition, producers and importers of products containing priority control substances need to report their products to the Ministry of Environment.
The picture below gives you an overview of amended K-REACH.
Important notes:
- The difference between registration and notification is that registration requires submission of hazard data. Notification only involves submission of some administrative info.
- Like EU REACH, K-REACH also restricts the use of certain hazardous chemical substances in consumer products and articles. [Read more...]
- If you manufacture chemicals or import chemicals, you not only need to comply with K-REACH, but also need to comply with Chemicals Control Act (CCA).
To understand the impact of Korea REACH regulation on your business, you only need to:
- Check if your products are within the scope of K-REACH;
- Identity your obligations under K-REACH;
Scope of K-REACH
| Within Scope |
|
| Out of Scope |
|
1.'New substance' is a substance that is not listed on Korean Existing Chemicals List(KECL). More info about KECLcan be found here.
K-REACH Pre-notification
K-REACH pre-notification will start from 1 Jan 2019 and end on 30 June 2019. It is required for all >=1t/y existing chemical substances manufactured in or imported to Korea. Only those pre-notified existing substances can benefit from registration grace periods, during which one can manufacture or import those pre-notified substances without full registrations.
K-REACH Registration
Under K-REACH, new substances must be registered prior to manufacture or import. >1t/y existing substances must be registered within the deadlines listed below.
- First 510 existing substances >=1t/y: 1 July 2018
- >=1000t/y and CMR substances >=1t/y: 31 Dec 2021
- 100-1000t/y: 31 Dec 2024
- 1-100t/y: 31 Dec 2030
Some substances and uses (i.e, R&D substance, export-only use, polymer of low concern) are exempt from full registration. However, you need to apply for confirmation on exemption.
Read more about K-REACH registration
Product Notification and Priority Control Substance
'Product' is a unique concept under K-REACH. A product means a mixture or an article used by consumers or a component of the mixture or the article that may expose consumers to chemical substances. Manufacturers and importers of products containing >0.1% and >=1t/y priority control substances shall submit product notification to the MoE.
How to Understand Priority Control Substance and Product Notification Requirement.
Old K-REACH vs Amended K-REACH
| Items | Old K-REACH | Amended K-REACH |
|---|---|---|
| Annual report |
|
|
| <100kg/y new substances |
|
|
| Pre-notification |
|
|
| Registration |
|
|
| Management of consumer chemical product and biocides |
|
|
| Product notification |
|
|
Industry's Obligations under Amended K-REACH
| Obligation | Target | Who/When |
|---|---|---|
| Pre-notification |
|
|
| Registration |
|
|
| Product Notification |
|
|
How to Comply with Amended K-REACH - for Foreign Companies
| Items | What to Do |
|---|---|
| Regulatory Check and Pre-notification |
|
| Registration |
|
| Product Notification |
|
| Restriction/Authorisation |
|
Comparison between EU REACH and K-REACH
| Items | EU REACH | K-REACH |
|---|---|---|
| Registration Target |
|
|
| Tonnage Band |
|
|
| Polymer |
|
|
| Only Representative |
|
|
| Pre-registration of Existing Substances |
|
|
| SVHC notification |
|
|
| Restriction/authorisation |
|
|
References
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Korea, REACH-like Regulation and Registration