How to Comply with Chemicals Control Act (CCA) in Korea
Little Pro on 2017-06-14
If you export a chemical product to South Korea, you not only need to comply with K-REACH, but also need to comply with Korean Chemicals Control Act (CCA). The CCA (previously toxic chemicals control act or TCCA) passed national assembly in May in 2013 and it comes into force in Jan 2015. It is a new law focusing on chemical reporting and chemical accident prevention. In this article, we will summarize the major obligations of chemical manufacturers and importers under CCA and how to prepare a letter of confirmation for CCA.
Obligations of Chemical Manufacturers and Importers
| Article | Description |
|---|---|
| Article 5 (Duties of Business Operators) |
|
| Article 9 (Confirmation of Chemicals) |
|
| Article 13 and 14 (Criteria for Handling Hazardous Chemicals and PPE) |
|
| Article 15 (Restrictions, etc. on Quantity of Hazardous Chemicals Displayed or Stored) |
|
| Article 16 (Labeling, etc. of Hazardous Chemicals) |
|
| Article 18 (Prohibition against Handling of Prohibited Substances) |
|
| Article 19 (Permission to Manufacture, Import, or Use Substances Requiring Permission, etc.) |
|
| Article 20 (Permission to Import Restricted Substances and Declaration for Import of Toxic Substances) |
|
| Article 23, 24, 25, 26 (Requirements on handling facilities) |
|
While CCA has also set out many obligations for government bodies, we will only focus on how to comply with CCA from an industry perspective and how to prepare the confirmation of chemicals under article 9.
Scope of CCA
The CCA does not apply to:
- Radioactive substances;
- Medicines and non-pharmaceutical of the Pharmaceutical Affairs Act;
- Narcotics under subparagraph 1 of Article 2 of the Act on the Control of Narcotics, etc.;
- Cosmetics and raw materials used for cosmetics under subparagraph 1 of Article 2 of the Cosmetics Act;
- Pesticides and technical ingredients under subparagraphs 1 and 3 of Article 2 of the Pesticide Control Act;
- Fertilizers under subparagraph 1 of Article 2 of the Fertilizer Control Act;
- Foods, food additives, appliances, containers, and packages under subparagraphs 1, 2, 4, and 5 of Article 2 of the Food Sanitation Act;
- Livestock feed under subparagraph 1 of Article 2 of the Control of Livestock and Fish Feed Act;
- Explosives under Article 2 (3) of the Control of Firearms, Knives, Swords, Explosives, etc. Act;
- Military supplies (excluding conventional items) under Article the Act on the Management of Military Supplies;
- Functional health foods under subparagraph 1 of Article 3 of the Functional Health Foods Act;
- Medical devices under Article 2 (1) of the Medical Devices Act;
- Toxic gases under the High-Pressure Gas Safety Control Act.
How to Comply with CCA
Firstly, you need to check the regulatory status of all substances in your chemical product, indicate wether your product contains any regulated substance below and submit the details to Korea Chemicals Management Association (KCMA) prior to manufacture or import.
- Existing chemical substances subject to registration under K-REACH;
- New chemical substances under K-REACH;
- Toxic substances;
- Substances requiring permission;
- Restricted substances;
- Prohibited substances;
- Substances requiring preparation for accidents.
You can click the following link, input the English name or CAS no. to check the regulatory status of each substance.
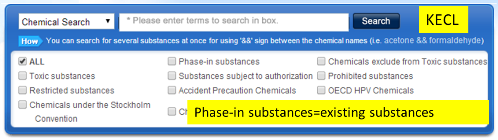
Secondly, you need to get additional approval if your product contains any regulated substance above. If your product contains any new substances or existing substances subject to registration, you need to register them with the national institute of enviromental research (NIER). If your product contains restricted or prohibited substances, you need to obtain prior permission from regional environmental office. If you manufacture or import toxic chemicals, you need to declare its kind and use to the regional environmental office.
Finally, you need to label your products correctly and ensure that your handling facilities meet all criteria or requirements set out in the CCA.
Letter of Confirmation for Manufacture and Importation
Manufacturer or Importer of chemicals shall submit a Written Confirmation of Details for Chemical Product to KCMA after conducting careful self-evaluation. Only one submission is needed for each product. If importers do not know detailed product composition, they could request suppliers to issue a signed confirmation letter confirming whether the product contains any regulated substance. The example of the confirmation letter is given below.
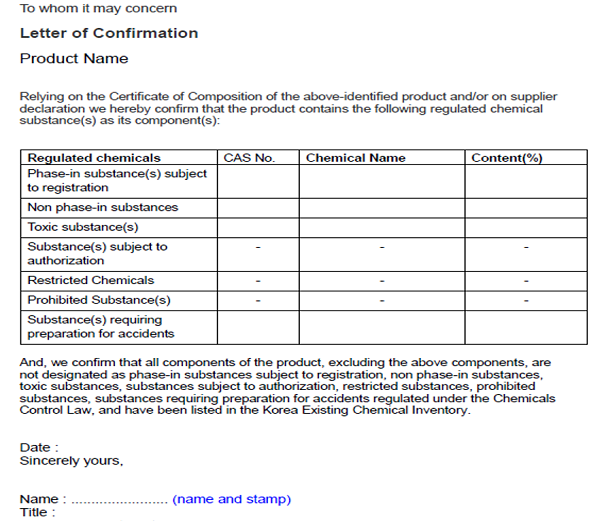
If you do not know how to check the regulatory status of each substance in your product, you could also apply for MoE to issue a confirmation certificate. You need to provide 100% product composition for this application.
Declaration of Imported Toxic Substances
If you intend to import a toxic substance into South Korea, you must disclose its kind and purpose to Regional Environmental Office.
References
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Korea, REACH-like Regulation and Registration