How to Comply with EU Biocidal Products Regulation (BPR)
Little Pro on 2017-11-30
In EU, the Biocidal Products Regulation (BPR) refers to Regulation (EU) 528/2012) concerning the placing on the market and use of biocidal products. It repeals the Biocidal Products Directive (Directive 98/8/EC) and comes into force on 1 Sept 2013. The regulation not only impacts manufacturers and importers of biocidal products, but also affects article producers who use biocidal products to treat their products. In this article, we will summarize how to comply with EU BPR regulation from different perspectives.
Main Requirements of EU BPR Regulation
The key requirement of BPR regulation is that all biocidal products require an authorisation by European Chemicals Agency (ECHA) before they can be placed on the EU market, and the active substances contained in that biocidal product must be previously approved.
The Biocidal Products Regulation (BPR) also sets rules for the use of articles treated with, or intentionally incorporating, one or more biocidal products. Articles can only be treated with biocidal products containing active substances approved in the EU. This also applies to articles manufactured outside of EU. Some treated articles require additional labelling.
Definition of Biocidal Products and Scope
The definition of biocidal active substance, biocidal products and treated article is listed as follows:
- Active substance: a substance or a micro-organism that has an action on or against harmful organisms.
- Biocidal produdcts: any substance or mixture, consisting of, containing or generating one or more active substances, with the intention of destroying, deterring, rendering harmless, preventing the action of, or otherwise exerting a controlling effect on, any harmful organism by any means other than mere physical or mechanical action.
- Treated article: Articles that have been treated with, or intentionally incorporating, one or more biocidal products.
Biocidal products covered by the BPR regulation are classified into 22 biocidal product-types, grouped in 4 main groups.
| Group | Product Types |
|---|---|
| Group 1 Disinfectant |
|
|
Group 2 Preservatives |
|
| Group 3 Pest control |
|
| Group 4 Other Products |
|
Note: It is very important to know your product type first to determine whether BPR regulation applies to your product or not. Take preservatives for example, BPR regulation does not apply to preservatives used in cosmetics and food because such uses are not covered by above product types. A treated article that has a primary biocidal function shall be considered a biocidal product.
List of Approved Active Substances
It is very important to find out if an active substance has been approved or not since only biocidal products containing approved active substances can be marked or used to treat articles. The list of approved active substances can be accessed below.
You can search the list by CAS no. or product types. Please see example below.
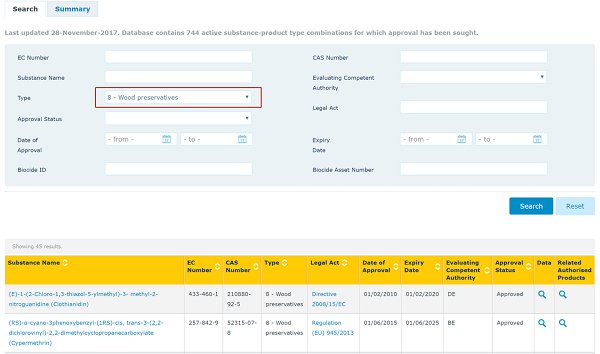
Biocidal products containing active substances in the Review Programme can be sold and used (subject to national laws) pending the final decision on the approval of the active substance (and up to 3 years after). Products containing new active substances that are still under assessment may also be allowed on the market where a provisional authorisation is granted.
Note: A supplier of an approved active substance cannot place the active substance on EU market unless the supplier itself is also listed in the list of active substances suppliers (Article 95 list).
List of Active Substances Suppliers (Article 95 List)
From 1 September 2015, a biocidal product cannot be made available on the EU market if the active substance supplier or product supplier is not included in the list of active substances suppliers (article 95 list).
The purpose of article 95 list is to ensure that the costs of the data on active substances are fairly shared. Only suppliers of active substances or biocidal products that have applied for inclusion in the list of active substances suppliers and submitted required info will be included in article 95 list.
ECHA regularly updates the Article 95 list. Non-EU companies can appoint an EU-based representative to apply for the inclusion in article 95 list. To download the article 95 list, please click the link below.
- Article 95 list - list of approved suppliers [Download]
Biocide Treated Articles and Labelling
The BPR requires that articles only be treated with biocidal products containing active substances that have been approved in the EU. This rule also applies to imported articles. Companies are also required to provide the consumers with information about the biocidal treatment of the article they are selling. If a consumer requests information about a treated article, the supplier must provide it free of charge within 45 days.
The BPR requires manufacturers and importers of treated articles to label treated articles when:
- a claim that the treated article has biocidal properties is made.
- it is required in the conditions of the approval of the active substance contained in the biocidal product used to treat the article.
The labeling of treated articles must be done according to both the Regulation on Classification, Labelling and Packaging (CLP) and the additional requirements in the Biocidal Products Regulation.
Note: If a treated article has a primary biocidal function, such an article needed to be authorised as a biocidal product.
How to Comply with EU BPR Regulation
| Role | How to Comply |
|---|---|
| Suppliers of biocidal active substances |
|
| Formulators or suppliers of biocidal products |
|
| Suppliers and manufacturers of articles |
|
Reference
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - EU, Biocidal Products Regulation