Pre-Manufacture and Pre-Importation Notification (PMPIN) of New Substance in Philippines
Little Pro on 2016-01-04
Pre-Manufacture and Pre-Importation Notification (PMPIN) for new substances was authorized by the Republic Act 6969 - Toxic Substances and Hazardous and Nuclear Waste Control Act of 1990. Manufacturers and importers of a new substance that is not listed on PICCS are required to notify DENR-EMB(*) of their intent to manufacture or import the new substance not sooner than 180 days and not later than 90 days.
*DENR-EMB:The Department of Environment and Natural Resources Environmental Management Bureau (DENR-EMB).
PMPIN Exemptions
The following substances are exempt from PMPIN.
- Chemicals and chemical substances included in PICCS;
- Small scale premises;
- Small quantity chemicals;
- Certain polymers and other substances exempt from PICCS requirements;
- Non-isolated intermediates;
- Articles;
- New chemicals manufactured exclusively for export;
Note: For small quantity of new substances(<1t/y) which are exempt from PMPIN, you must apply for Small Quantity Importation Certification (SQI). Even for substances listed listed on PICCS, you may apply for PICCS certification(not a compulsory requirement).
The picture below shows how to determine if a substance requires notification and what type of certificate is applicable.
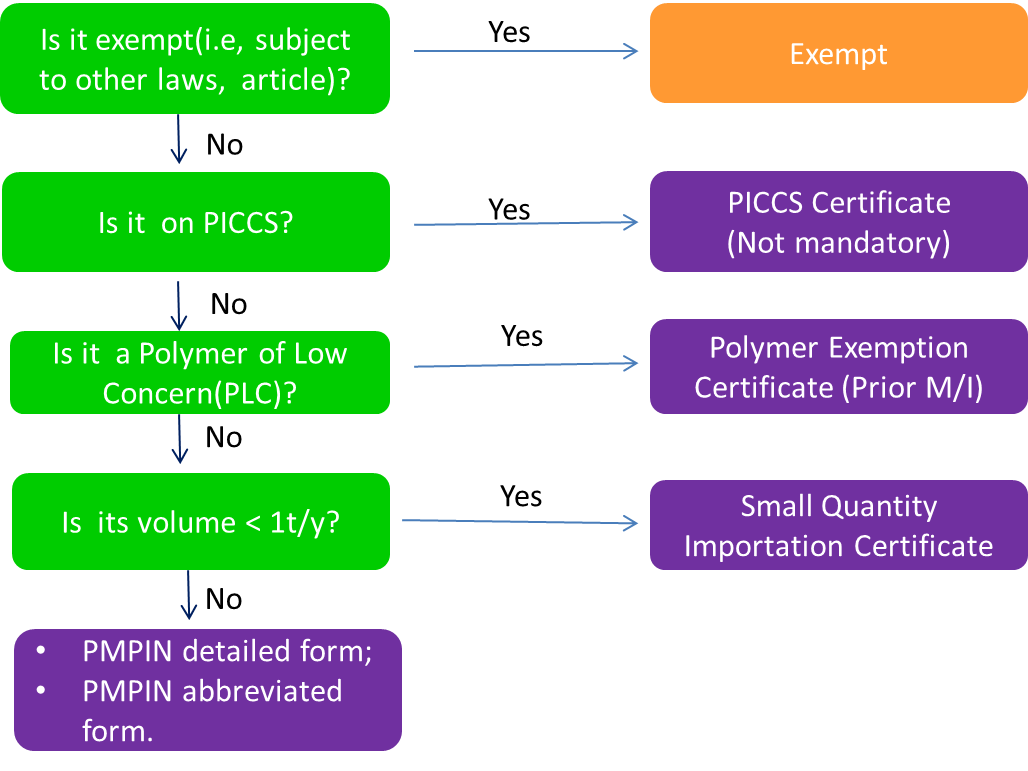
PMPIN: Types of Notification and Data Requirements
There are two types of PMPIN for new substances manufactured or imported with volume(>=1,000kg/y): abbreviated form and detailed form.
| PMPIN Abbreviated form |
|
| PMPIN Detailed form |
|
Information Required for PMPIN
- Application form and notarized copies;
- MSDS/SDS In ISO 11014 or GHS format
- Environmental effect of the chemicals (if not included in MSDS/SDS);
- Specific uses;
- Annual quantity of substances to be manufactured or imported;
- Request for Confidential Business Information (CBI) - if needed
- Payment of processing fee(~48 USD for abbreviated form or 84 USD for detailed form)
- GLP test reports with physio-chemical info, toxicological info and eco-toxicological info(for detailed form only).
Notice of Commencement after PMPIN
Once a notification has been granted, a registrant must submit a Notice of Commencement (NOC) of Import Form within 30 days since the first import. Only after submission of this form will the new substance be added to PICCS. The new chemical substance will be added to the PICCS 1 year after the NOC has been submitted unless registrants have applifed for CBI protection.
Small Quantity Importation Certification
Small Quantity Importation Certification(SQI) is required prior to the importation of small quantity of new substances (less than 1000 kg/year) into the Philippines. The following info is required for application of SQI.
- Completed application form and notarized copies
- (M)SDS in ISO11014 or GHS format
- Letter of request for SQI clearance
- Official receipt of processing fee (~11 USD per substance)
Polymer Exemption Certification
Companies shall apply for polymer exemption certificate if their new polymers meet one of the following criteria.
- all of the monomers ( >=2%w/w) are listed on PICCS; or
- 2 or more of the top (top by weight) monomers are included in the definition of another polymer already in PICCS.
The information required includes a letter of request, SDS and detailed composition list. New polymer substances which do not meet above criteria must go through PMPIN or SQI process.
PICCS Certification
The purpose of PICCS certification is to show the Bureau of Customs or other authorities that a substance is indeed listed on PICCS. Companies may apply for PICCS certification for their substances by submitting the following info
- Completed PICCS application form and notarized copy
- (M)SDS in ISO11014 or GHS format
- Letter of request for PICCS certification
- Official receipt of processing fee (~10 USD per substance)
GHS in the Philippines
Philippines has already implemented GHS in workplace through the "Guidelines for the Implementation of Global Harmonised System (GHS) in Chemical Safety Program in the Workplace" issued in 2014. GHS SDSs and labels are required for hazardous substances and mixtures in workplace from 14 March 2015.
Read more about GHS in the Philippines.
Reference & Resources
Click here to access all references and resources for Philippine including the English translation of regulations, regulatory lists and useful links to the websites of competent authorities.
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Philippine, REACH-like Regulation and Registration