How to Comply with Food Contact Regulations in USA
Little Pro on 2017-03-03
If you place a food contact material (i.e, paper, adhesives, plastics) or article on the US market, you need to determine whether it complies with food contact regulations in USA or not. If you sell a substance that is used to produce food contact materials, you will need to know whether its use has been cleared by FDA or not. In this article, we will show you how to check the regulatory status of your food contact material (including additives) and how to demonstrate compliance with food contact regulations in USA. Examples of FDA letter of guaranty will be given in the end of this article.
Overview of Food Contact Regulations in USA
In the US, the regulatory status of a food contact material or article is determined by the regulatory status of each individual substances that constitute the material or article. A substance intended for use as a component of materials used in manufacturing, packaging, transporting, or holding food (also known as food contact substance) is regulated as indirect food additive under the Food Drug and Cosmetic Act (FD&C Act) and Title 21 Code of Federal Regulations (21 CFR). A food contact material or article is compliant only if all food contact substances contained have got FDA clearances.
It should be noted that only those food contact substances that migrate to the food (i.e., expected to become a component of the food) are regarded as indirect food additives and require FDA pre-market clearance. For example, if a substance is separated from food by a functional barrier and prevented from migration, the substance will not be considered as indirect food additives, and thus not require FDA clearance.
Types of FDA Clearances
The table below summarizes general provisions for indirect food additives and the main types of FDA clearances.
| Regulation/Type | Description |
|---|---|
|
21 CFR 174 General provisions applicable to indirect food additives |
|
|
21 CFR 175, 176, 177, 178 and 179 Positive list of substances used to manufacture certain types of food contact materials. |
When using substances on the positive lists (21 CFR 175-179) , companies must also comply with prescribed limitations if there are any.
|
|
21 CFR 181, 21 CFR, 182-186, 21 CFR 170.39 Special types of clearances.
|
Generally Recognized As Safe (GRAS) (21 CFR, 182-186) Substances generally recognized as safe (GRAS) can be safely used as components of articles that contact food under conditions of good manufacturing practice. A list of GRAS notices with FDA's response can be searched here. Please note that not all substances that are GRAS are listed in 21 CFR or covered by a GRAS notification. Prior Sanctioned Substances (21 CFR 181) Prior sanctioned substances are those substances whose use in contact with food is the subject of a letter issued by FDA or USDA before 1958 offering no objection to a specific use of a specific substance. The list of pre-sanctioned substances can be accessed here. Threhold of Regulation (21 CFR 170.39) A substance used in a food contact article may be exempted by FDA from the need of an FCN or a petition (regulation) as a food additive if the use has been shown to result in a very low concentration (0.5 ppb). More info can be found here. Food Contact Notification (FCN) Companies who would like to use a new food contact substance in food contact materials may submit a notification to FDA and apply for pre-market approval. Please note that a food contact notification (FCN) is only effective for the manufacturer or supplier identified in the notification. |
Consolidated Database of Authorized Food Contact Substances
If you would like to know whether a substance has been authorized for food contact use or not, the quickest way is to search the following 3 lists . It is a lot easier than searching individual 21 CFR lists or GRAS notices.
- Step 1: Search the consolidated list of authorized indirect food additives (21 CFR 175 - 178)
- Step 2: Search the list of GRAS notices
- Step 3: Search the list of prior-sanctioned food ingredients
The picture below is a screenshot of FDA's consolidated database of authorized indirect food additives under 21 CFR 175-178.
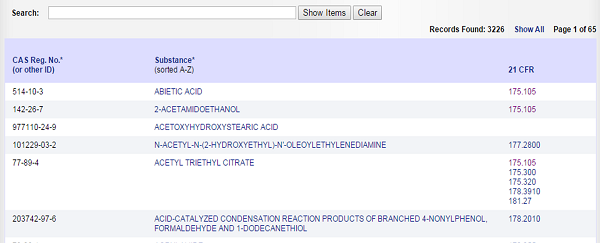
Please note that the presence of a substance in above list does not mean that the substance has been authorized for use in all food contact materials. When reviewing your composite formulations to determine compliance, consider each authorization to be composed of three parts: the identity of the substance, specifications including purity or physical properties and limitations on the conditions of use. You can get more info about the conditions of use and limitations of a substance by clicking corresponding 21 CFR part no.
FDA Letter of Guaranty
Unlike in EU, declaration of compliance (DOC) is not compulsory for food contact additives or food contact materials in the USA. However, customers may ask for a letter of guaranty from manufacturers certifying that a particular substance or material is acceptable for the intended food-contact use.
Section 303 of the Federal Food, Drug, and Cosmetic Act (the Act) states that no person shall be subject to penalities for having received, or proffered delivery of, adulterated or misbranded food additives if he has established a good faith guarantee from whom he received the articles.
A letter of guaranty is a self-declaration regarding product compliance. There is no standard format. Several real examples of FDA letter of guaranty are given here for your reference.
- FDA Letter of Guaranty by The Box Maker
- FDA Food Contact Compliance Declaration by UPM
- Regulatory Compliance Statement for Food Contact Materials by Chemours
Food Contact Notification
If your substance has not been authorized for food contact use, you may submit a food contact notification to the FDA. A notification for a new food contact substance or expanded use of an existing substance must contain sufficient information to demonstrate that the substance is safe for the intended use. FDA has drafted guidance on chemistry, toxicology, and environmental information requirements for food contact notification. Read more...
References
- Determining the Regulatory Status of Components of a Food Contact Material - by FDA
- US FDA Regulation of Plastic Used in Food Packaging by Tom Dunn
- FDA Regulation of Adhesives in Food Packaging - by George G. Misko
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..

Tags: Topics - Food Contact, Food Contact Regulations