Swiss Chemicals Ordinance (ChemO)
Little Pro on 2016-01-05
Since Switzerland is not a member of the EU or the European Economic Area (EEA), EU REACH regulation does not apply here. Switzerland has its own chemical regulations adopting REACH-like registration requirements, but only on new substances placed on the Swiss market.
The provisions governing the obligation to notify, declare and register new substances are contained in its Chemicals Ordinance on Protection against Dangerous Substances and Preparations (known as ChemO). However, the Swiss Chemicals Ordinance (ChemO) governs more than just new substances. It has been amended several times. The latest edition is the 4th revised version which entered into force on 1 December 2012.
Overview and Main Requirements of Swiss Chemicals Ordinance (ChemO)
The picture below shows how chemicals are regulated under the Swiss Chemicals Ordinance (ChemO):
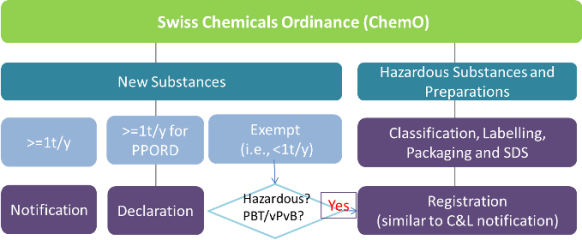
Under the Swiss Chemicals Ordinance, all >=1t/y new substances must be notified or declared in Switzerland before they are placed on the market, even if they are already registered under EU REACH. The chemical authority is the Notification authority for chemicals of the Swiss Federal Office of Public Health.
The ChemO has also adopted GHS by requiring suppliers to classify, label and package hazardous substances and preparations in accordance with EU CLP regulation. In the meantime, manufacturers and importers are required to submit the classification and labelling info of hazardous substances (including <1t/y new substances and exempt new substances) and preparations to the Swiss authority within 3 months after first placing them on the Swiss market. This obligation is called registration. It is similar to C&L notification under CLP regulation.
In addition to that, the ChemO has introduced REACH SVHC list into Switzerland and imposed a duty on suppliers of articles which contain substances on the SVHC list in a concentration above 0.1% (w/w) to provide sufficient information to their customers to allow safe use of the articles.
Definition of a New Substance and Swiss Chemical Inventory
A new substance is defined a substance that is not listed on EINECS (European Inventory of Existing Commercial Chemical Substances). This inventory includes chemical substances deemed to be on the European Community market between January 1, 1971 and September 18, 1981.
Please note that there is no official Swiss chemical inventory. Switzerland also uses EINECS as their existing chemical substance inventory. A substance that is listed on EINECS may still require registration under the Swiss Chemicals Ordinance. For more info, please refer to ChemO registration part.
Differences between Notification, Declaration and Registration under ChemO
| Notification |
|
| Declaration |
|
| Registration |
|
*Decisive quantity: This is the higher one of the annual quantity of a substance placed on Swiss market and the total quantity introduced to the EEA in a year. For example, if an American company plans to export 99 tons of a new substance into the EEA, among which 5t is imported into Switzerland, the American company will need to submit a 10-100t technical dossier to Swiss authority by appointing an only representative.
New Substance Notification Scope and Exemptions
A manufacturer or an importer must notify a new substance before it is placed on the market for the first time in Switzerland. Notification applies to a substance on its own, in a mixture or intended to be released from an article under normal conditions of use. For polymer, this notification applies to monomers. Similar to EU REACH, a company based outside of the Switzerland can appoint a Swiss only representative to notify.
The following new substances are exempt from new substance notifications in Switzerland under the ChemO:
- Chemical substances that are outside the scope of ChemO (i.e., in-transit substance, medicines, food stuffs, weapon, wastes);
- Polymer or substance contained in a polymer with a concentration below than 2%;
- Substances listed in the no-longer polymer list (NPL);
- New substances placed on Swiss market with volume less than 1t/y;
- <1t/y new substances placed on Swiss market purely for scientific R&D;
- Substances used exclusively as raw materials, active ingredients or additives in food, therapeutic products and animal feed;
- Substances that are used exclusively as active ingredients in crop protection products and biocidal products;
- Substances acquired in Switzerland;
- Intermediates, provided that they are not monomers;
- Substances listed in REACH annex V (by-products, naturally occurring substances, impurities, etc.);
- Substances for PPORD purpose(**);
*PPORD: This exemption needs to be declared to authority. This exemption is usually limited to a quantity required for the purpose and a period not exceeding five years. For more info, please refer to new substance declaration part.
New Substance Notification Data Requirements
Data requirement for new substance notification under ChemO is the same as EU REACH registration. Higher data package is required for higher volume substances. A notification fee also needs to be paid.
Luckily, the Swiss Authority accepts REACH registration dossier in IUCLID 5 format. For substances registered under EU REACH after 1 June 2008, it is not mandatory to send in copies of full test reports. A fully summary in IUCLID 5, meeting the criteria of a robust study summary, is sufficient. A letter of access is required if a late notifier wishes to share the data submitted by a previous registrant.
New Substance Declaration under ChemO
For >=1t/y new substances which are placed on the market solely for the purpose of product- and process-orientated research, a manufacturer, importer or only representative needs to apply for applications for exemption at least 30 days before he/she intends to place the substance on the market for the first time.
This exemption can only be claimed if the substances meet the following conditions:
- exclusively for the purpose of product- and process-orientated research and development,
- in quantities no greater than those necessary for the stated purpose, and
- for a period not exceeding five years (may be extended upon approval);
Compared to full notification, new substance declaration only requires information on legal entity, substance identity, uses and expected quantity.
Chemical Registrations under ChemO
Manufacturers or importers must register the following hazardous substances or preparations with the Notification Authority within 3 months after first placing them on the Swiss market.
- Substances and preparations meeting CLP/GHS classification criteria;
- PBT or vPvB substances;
- Substances listed in the annex 7 of ChemO (SVHC candidate list);
- Preparations containing at least one hazardous ingredient that is dangerous to human health or to the environment in an individual concentration>=1%w/w (non-gases) or >0.2% by volume(for gases);
- Preparations containing at least one PBT or vPvB substance in an individual concentration>=0.1%w/w;
- Preparations containing at least one SVHC in an individual concentration>=0.1%w/w;
- Preparations containing at least one substance for which a workplace exposure limit has been laid down in Europe.
The purpose of this registration is to provide the Swiss authority with information on the classification and labelling of hazardous chemicals placed on the Swiss market. It is similar to C&L notification under the EU CLP regulation. Required info is very simple and mainly includes substance identity, composition of preparation, intended uses and classification and labelling.
Reminder: Unlike new substance notification or declaration, this registration needs to be done on a per product basis.
ChemO Registration Exemptions
The following chemicals are exempt from registrations under ChemO:
- Chemical substances that are subject to other laws (i.e., medicines, food stuffs, weapon, wastes, ammunition, fertilizer, therapeutic products, etc.);
- Intermediates;
- Substances and preparations solely for R&D;
- Substances obtained in Switzerland;
- Gas mixtures consisting exclusively of registered gases;
- Preparations <100kg/y and intended exclusively for professional users;
Note: A notified or declared new substance does not require registration.
Handling of Substances of Very High Concern (SVHC)
Substances on REACH SVHC list will be added to the Annex 7 of ChemO. Anyone who commercially supplies an article containing a substance of very high concern in a concentration greater than 0.1 % by weight must provide the customer with the following information:
- the name of the substance concerned;
- all other information required to allow safe use of the object;
This information must be provided free of charge to professional or commercial users without being requested or consumers within 45 days upon request.
Note: The restriction of the marketing and uses of certain substances, preparations and articles are governed by another ordinance Chemical Risk Reduce Ordinance (ORRChem).
More Readings
- Swiss Chemical Risk Reduction Ordinance (ORRChem);
- GHS in Switzerland;
- EU REACH Regulation;
- EU CLP Regulation;
- REACH SVHC List
Reference & Resources
Click here to access all references and resources for Switzerland including the English translation of regulations, regulatory lists and useful links to the websites of competent authorities.
Having Questions?
We do not provide consultancy services. If you have questions or need any help, please contact our sponsor. You may also find an expert in CSP business directory below. If you are a consultant, you may get yourself listed in CSP business directory (free) or sponsor this page to leave your contact info on this page..
